
Spring, male hormones and health: what you need to know
04.03.2025

A delegation from the Cuban Ministry of Health visited Belarus to launch cooperation in the pharmaceutical sector
04.03.2025

10 years we take care of the main thing. Presentation film
24.10.2022

We take care of the main thing. Presentation film
24.10.2022

Рresentation film 2018
24.10.2022
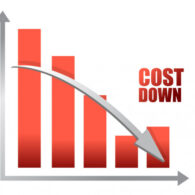
Rivaksan has become more affordable
20.12.2021

Rivaksan-Innovation of the Year at the 2021 Eurasian Summit
15.10.2021
The results of the competition “Competence – 2020” among accredited subjects and technical experts on accreditation have been summed up!
Every year, since 2011, within the framework of the National Accreditation System of the Republic of Belarus, the State Enterprise “Belarusian State Accreditation Center” organizes and conducts the Competition “Competence”. Its purpose is to identify and encourage active participants in accreditation activities who are professionals and leaders in conformity assessment and accreditation activities.
The winners of the competition are given the right to use the competition mark in their advertising materials. This opportunity contributes to attracting new partners and customers, maintaining and increasing the existing market share, which leads to an increase in profits and, accordingly, opens up new business opportunities.
The Quality Control Department of the State Enterprise “ACADEMPHARM” was recognized as the winner of the “Competence – 2020” competition in the “Testing Laboratory” nomination.
On September 29, 2021, within the framework of the Belarusian Industrial and Innovation Forum 2021, a solemn ceremony of awarding the winners of the “Competence – 2020” competition was held.
Presenting awards to the winners of the competition, the director of the Belarusian State Accreditation Center Evgeny Viktorovich Berezhnykh noted that the accreditation body marks the most successful organizations that have demonstrated a high level of competence, improve their activities taking into account the international practice of world leaders and make a significant contribution to ensuring an objective assessment of the conformity of products and services provided.




